The place to find all of the legal abortion clinics in the United Kingdom
Contraception
You will find all the information that you should know about contraception here. We tell you about all the contraception that you need to know about in order to find the most suitable for you.
Written by www.abortionclinicsinuk.co.uk in Contraceptives on the 18/11/2015
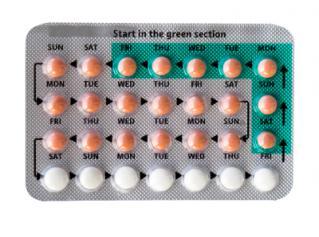 Birth Control Packaging Error
Birth Control Packaging Error
In 2011 there was a recall on eight oral contraceptives.
"This caused women to take placebo pills during the week they should have been taking hormone pills, leaving them at risk for conception",
Endo Pharmaceuticals said that it had been able to confirm that one blister pack was defective. This meant that "This caused women to take placebo pills during the week they should have been taking hormone pills, leaving them at risk for conception".
The women affected are now seeking millions in damages due to the suffering, pain, medical costs and price of raising the children that they had not planned to have.
A lawsuit has been filed in Philadelphia which is awaiting the response of the pharmaceutical companies.
Those products included in the recall were Cyclafem, Emotiquette, Glidess FE, Orsythia, Previfem and Tri-Previfem. More than 3.2 million blister packs of pills were recalled.
Source: www.abortionclinicsinuk.co.uk
- Normas de participación
- > Esta es la opinión de los internautas no de Clinicasabortos.mx
- > No se admiten insultos ni faltas de respeto
- > No esta permitido hacer comentarios contrarios a las leyes Mexicanas o injuriables
- > Clinicasabortos.mx se reserva el derecho a eliminar los comentarios que considere ofensivos o fuera de tema
Most popular FAQs
Questions by recent users
- Is there anyway I can find out about a previous abortion I have had?
- hello, am not sure but I think I might be pregnant as i had unprotected sex during my fertile days the test says negative but my tummy hasn't stopped hurting
- Please can you contact me to discuss adding our services to your site - see www.nupas.co.uk. We are contracted by 60 CCGs to provide TOPs across the UK, Many thanks
- I have missed my period but the pregnancy test is negative
- Is an abortion with pills reversible after the first pill?






